Silver has a density of 10.49 grams per cubic centimeter. This makes it one of the densest precious metals.
Silver’s high density is a key characteristic that distinguishes it from other metals. This dense nature makes it ideal for various industrial applications, including electronics and jewelry. The metal’s high electrical conductivity and thermal conductivity make it valuable in manufacturing.
In the world of jewelry, silver’s density contributes to its weight and feel, enhancing its appeal. Investors also appreciate silver’s density as it provides tangible value in a compact form. Understanding silver’s density is crucial for industries that rely on precise measurements and material properties. Overall, silver’s density plays a significant role in its widespread use and value.

Credit: nootka-jewelry.com
Introduction To Silver’s Density
Silver is a fascinating metal with many uses. It is known for its shiny appearance and unique properties. One important property of silver is its density. Understanding silver’s density helps us in many fields, such as jewelry making, electronics, and chemistry.
What Is Density?
Density tells us how much mass fits in a given volume. Think of it as how tightly matter is packed together. In simple terms, density is mass divided by volume. We measure density in grams per cubic centimeter (g/cm³).
Basic Properties Of Silver
Silver is a precious metal with the chemical symbol Ag. It is well-known for its shiny, white appearance. Here are some basic properties of silver:
- Atomic Number: 47
- Atomic Mass: 107.87 u
- Melting Point: 961.8°C
- Boiling Point: 2162°C
- Density: 10.49 g/cm³
Silver is very conductive. It is used in many electrical applications. Its high density makes it valuable in various industries.
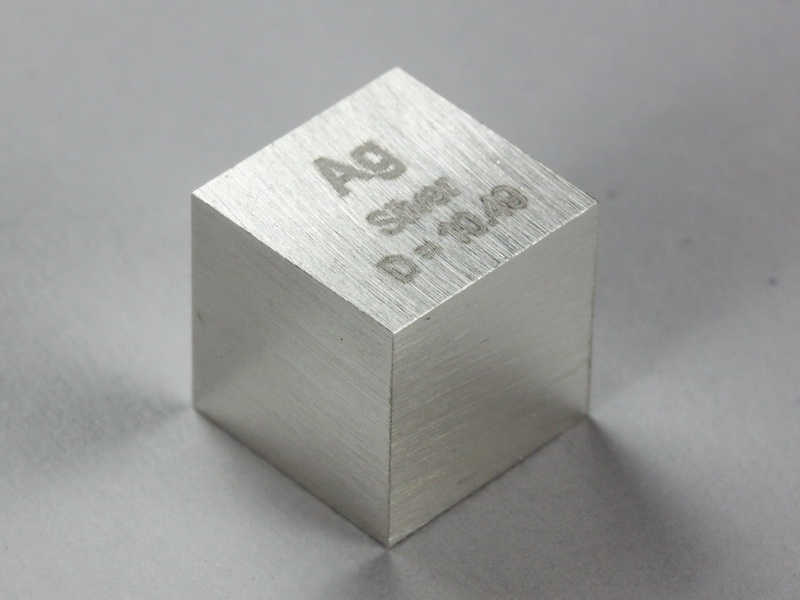
Credit: www.smart-elements.com
Measuring Silver’s Density
Understanding the density of silver is essential for various applications. It helps in jewelry making, industrial processes, and scientific experiments. Silver’s density is a measure of how much mass is contained in a given volume.
Standard Units
The density of silver is usually measured in grams per cubic centimeter (g/cm3). In the metric system, it is a standard unit of measure. Silver’s density can also be expressed in kilograms per cubic meter (kg/m3).
| Unit | Equivalent |
|---|---|
| 1 g/cm3 | 1000 kg/m3 |
| 1 kg/m3 | 0.001 g/cm3 |
Silver has a density of about 10.49 g/cm3. This makes it one of the heavier metals used in various industries.
Measurement Techniques
There are different ways to measure the density of silver. Here are some common techniques:
- Water Displacement Method: Submerge silver in water. Measure the water displaced. This helps calculate the volume.
- Archimedes’ Principle: Weigh silver in air, then in water. Use the weight difference to find density.
- Hydrostatic Balance: Use a specialized balance to measure density directly.
Each method has its own advantages. The water displacement method is simple but effective. Archimedes’ principle is accurate for irregular shapes. The hydrostatic balance is precise and quick.
Using these methods, you can accurately determine the density of silver. Accurate density measurement is crucial in both scientific research and industrial applications.
Factors Affecting Silver’s Density
Silver is a precious metal known for its shiny appearance and conductivity. Its density can vary based on several factors. Understanding these factors helps in various industrial and scientific applications.
Temperature Influence
Temperature changes can affect silver’s density. As temperature increases, silver expands. This expansion decreases its density. Conversely, lowering the temperature causes silver to contract. This contraction increases its density.
Impurities And Alloys
Silver is rarely pure. It often contains impurities or is mixed with other metals. These impurities can affect silver’s density. For example, adding copper to silver creates sterling silver. This alloy has a different density than pure silver.
| Type of Silver | Density (g/cm³) |
|---|---|
| Pure Silver | 10.49 |
| Sterling Silver | 10.36 |
Impurities can also affect the density. Even a small amount of foreign material can change it. This is important in jewelry and electronics. Ensuring the right density is crucial for quality.
Comparing Silver With Other Metals
Silver is a precious metal known for its unique properties. One key property is its density. But how does silver’s density compare with other metals? Let’s explore this by comparing silver with gold and platinum.
Silver Vs. Gold
Gold is denser than silver. The density of gold is 19.32 g/cm3. Silver’s density is 10.49 g/cm3. This means gold is almost twice as dense as silver.
Here’s a quick comparison:
| Metal | Density (g/cm3) |
|---|---|
| Silver | 10.49 |
| Gold | 19.32 |
Silver Vs. Platinum
Platinum is another dense metal. Platinum’s density is 21.45 g/cm3. Comparing this to silver, platinum is more than twice as dense.
Here’s how they stack up:
| Metal | Density (g/cm3) |
|---|---|
| Silver | 10.49 |
| Platinum | 21.45 |
These comparisons show silver is less dense than both gold and platinum. This property affects silver’s uses in various industries.
Applications Of Silver’s Density
Understanding the density of silver opens up a world of applications. Its unique density influences its use in various fields. Let’s explore some of these exciting applications.
Industrial Uses
Silver’s density makes it perfect for industrial applications. It is used in high-precision instruments. These include electrical contacts and conductors. Silver’s density ensures durability and high conductivity.
In the medical field, silver is used for its antibacterial properties. Dense silver coatings on medical devices prevent infections. The density of silver also enhances radiation shielding. It protects against harmful radiation in nuclear reactors and laboratories.
| Application | Reason |
|---|---|
| Electrical Contacts | High Conductivity |
| Medical Devices | Antibacterial Properties |
| Radiation Shielding | Protection from Radiation |
Jewelry And Art
Silver’s density plays a crucial role in jewelry making. It allows for intricate designs and durability. Dense silver jewelry is valuable and long-lasting.
Artists appreciate silver’s density for sculptures and decorative pieces. It provides weight and a luxurious feel. Silver’s shine and density make it a favorite in art and design.
- Intricate Designs – Allows for detailed craftsmanship.
- Durability – Ensures long-lasting pieces.
- Luxurious Feel – Adds value to art and sculptures.

Credit: www.researchgate.net
Frequently Asked Questions
What Is The Density Of Silver?
The density of silver is approximately 10. 49 grams per cubic centimeter.
How Does Silver’s Density Compare To Gold?
Silver is less dense than gold. Gold has a density of about 19. 32 grams per cubic centimeter.
Why Is Silver’s Density Important?
Silver’s density is crucial for applications in jewelry, electronics, and industrial uses where weight and volume matter.
Does Temperature Affect Silver’s Density?
Yes, temperature can affect silver’s density. Higher temperatures cause expansion, slightly reducing density.
Is Silver Denser Than Copper?
Yes, silver is denser than copper. Copper has a density of about 8. 96 grams per cubic centimeter.
How Is Silver’s Density Measured?
Silver’s density is measured using a balance to find mass and a graduated cylinder or displacement method to find volume.
Conclusion
Understanding the density of silver enhances our knowledge of its properties and uses. Silver’s high density makes it valuable in various industries. Knowing this can assist in making informed decisions. Whether in jewelry or electronics, silver’s density plays a crucial role.
Keep these facts in mind when considering silver for any application.
